BY LAURA OLSON
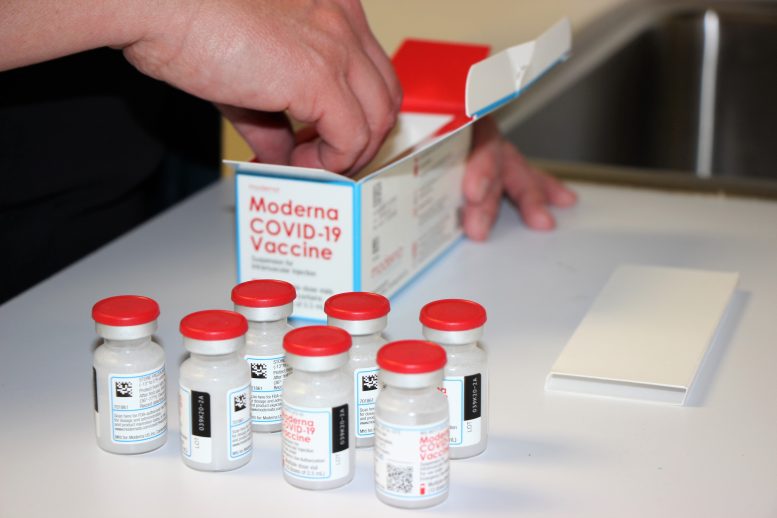
WASHINGTON — Tens of millions of additional Americans are now eligible to receive a booster dose of one of the COVID-19 vaccines, after federal health officials gave the green light late Thursday to follow-up doses of the shots made by Moderna and Johnson & Johnson.
Anyone who received the one-shot J&J vaccine is now eligible for a second dose at least two months after their shot.
Moderna recipients who are over age 65 or at higher risk due to their medical condition or work environment also are eligible for a partial third dose at least six months after their second shot.
Those who received Pfizer’s vaccine already were eligible to receive a booster dose.
The recommendation from the Centers for Disease Control and Prevention also allows individuals to receive a booster dose from a different company than the one that manufactured the initial vaccine that they received.
Some have wanted a different followup dose due to adverse reactions to a certain vaccine. Others have been concerned about the J&J shot, which studies have shown to have a lower efficacy against infection compared to the ones from Pfizer and Moderna.
The three vaccines against COVID-19 “are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating delta variant,” Dr. Rochelle Walensky, director of the CDC, said in a statement Thursday night.
A Pfizer study released Thursday showed that a booster dose of the company’s vaccine was 95.6% effective in the trial of more than 10,000 participants.
The newly authorized booster shots come as the sharp spike in infections and deaths caused by that delta variant has begun to wane.
But the country is still seeing about 75,000 new cases every day, and about 1,300 COVID-19-related deaths, according to CDC tracking data.
***
Also from Ohio Capital Journal:
Commentary: An infectious disease expert explains new rules on ‘mix-and-match’ vaccine booster shots
By Glenn J. Rapsinski, University of Pittsburgh Health Sciences
Many Americans now have the green light to get a COVID-19 vaccine booster – and the flexibility to receive a different brand than the original vaccine they received.
On the heels of the Food and Drug Administration’s Sept. 22, 2021, emergency use authorization of a third dose – or “booster shot” – of the Pfizer-BioNtech vaccine for certain Americans, on Oct. 20, the agency also gave emergency authorization to a third Moderna shotand a second dose of the Johnson & Johnson vaccine.
On Oct. 21, the Centers for Disease Control and Prevention also recommended these vaccinations in light of the FDA’s authorization. The CDC’s signoff will make the Moderna booster shot available to people 65 and older, younger adults at higher risk of severe COVID-19 due to medical conditions and those who are at increased risk due to their workplace environment. People are now eligible for the Moderna booster six months after completion of their original series – as is already the case for the third Pfizer shot. The authorization made all Johnson & Johnson vaccine recipients eligible for a second shot two months after the initial dose.
Notably, the FDA and CDC also authorized a “mix-and-match” strategy, enabling eligible Americans to get a booster shot from a brand different from their original vaccine. READ MORE
‘Aisha’s Law’ on domestic violence passed out of committee
A proposed law named for a Shaker Heights woman who lost her life to domestic violence was approved by a House committee on Thursday.
Aisha’s Law was passed out of the House Criminal Justice Committee after receiving even more testimony on its importance, and despite arguments against it by a criminal defense association.
The bill was named after Aisha Fraser, who was killed by the father of her children, Lance Mason, a former state legislator.
“We are one step closer to honoring Aisha’s life and light, and so many others,” said bill cosponsor state Rep. Janine Boyd, D-Cleveland Heights, in a statement after the bill was passed out of committee.
House Bill 3 would expand the legal definition of domestic violence to include strangulation and require more in-depth rules and procedures for law enforcement officers, including the implementation of a “lethality assessment” when responding to a domestic violence situation. READ MORE
Cuyahoga grand jury declines to charge officer in January shooting
East Cleveland police officer Larry McDonald will not face charges in the January shooting that left Vincent Belmonte dead. Ohio Attorney General Dave Yost announced the Cuyahoga County grand jury’s decision Friday.
The incident stems from a traffic stop on the morning of Jan. 5, this year. McDonald was on traffic patrol, training fellow officer Dillon Crosier. According to state Bureau of Criminal Investigation agents, McDonald said an equipment violation was the pretext for the traffic stop.
“Sgt. McDonald stated that the vehicle he observed had a loud muffler and Sgt. McDonald advised that he could see the cracked windshield as well,” the report reads.
While the car was pulled over, McDonald says they ran the car’s license plate. He told investigators it was a “red flag” when the report came back as a dealer plate, and he believed the car might flee because of how the driver was checking his rear and side view mirrors. READ MORE