By DAVID DUPONT
BG Independent News
Superbugs may sound like a new summer time horror movie, but the dangers they pose are real.
This past semester Dr. Shannon Manning, from Michigan State University, presented “Superbugs! Antibiotic Resistance Matters,” the keynote address of the Ned Baker Public Health Symposium.
The talk, despite its sensational title, was aimed at those in public health. The talk delved deeply into biological mechanisms as Manning explained the rapid evolution of pathogens that are resistant to antibiotics and other treatments.
The use of antibiotics, she said, goes back to well before people knew what they were. They were present in ancient beer, an unpalatable brew akin to liquid bread dough. Egyptians used honey and lard to treat wounds because of the anti-microbial properties.
But superbugs arose only after scientists understood these properties and created drugs. This launched an evolutionary war between the drugs meant to cure diseases and the pathogens that cause diseases.
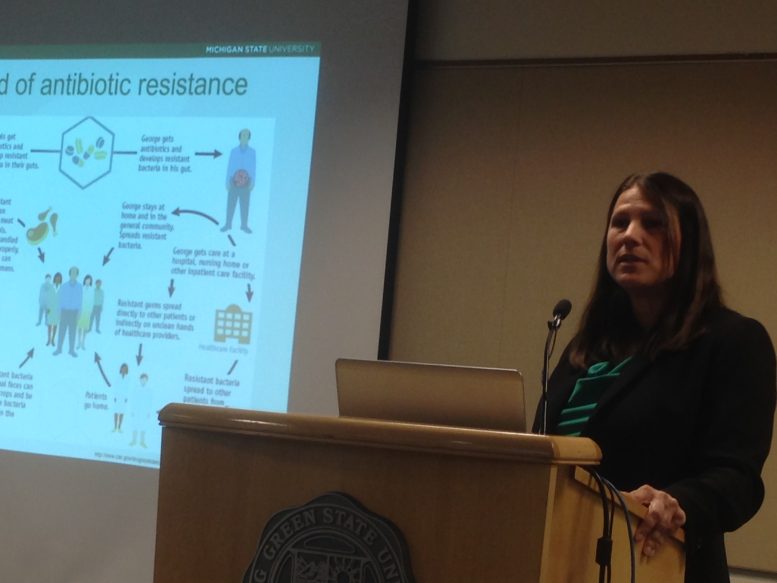
While the antibiotic kills most of the pathogens, a few cells immune to the antibiotic survive, and thrive, creating new strains immune to the antibiotic.
That led to the emergence of superbugs.
“They can cause high rates of morbidity and mortality in human populations and also are causing high rates of disease in animal populations,” Manning said “Some of these superbugs tend to be more virulent causing more severe infections.”
That leads to high rates of death and long-lasting health problems, she said. Patients end up “sicker for very long period of times.”
“We do have a set a resistant pathogens that cannot be killed by any of known drugs that we have,” Manning said. “Many of these pathogens and others have developed resistance to many types of antibiotics. These are increasing in number.”
None of this should have been surprising. Alexander Fleming the scientist who first isolated penicillin warned of the drug’s of overuse, Manning said.
Unheeded, his warning was proven true quickly. At first penicillin was considered a miracle drug. Staph aureus killed 70 percent of its victims before the drug was discovered. Those fatalities dropped dramatically once people started taking penicillin. That prompted increased use of penicillin. Nature reacted, and drug-resistant strains evolved. Death rates rose again.
Hospitals are battlegrounds. They have many patients who already have compromised immune systems and are targets for these new drug-resistant pathogens.
The war between drugs and pathogens was engaged with drug resistant strains emerging as soon as a new drug was developed. That led to the evolution of the superbugs, pathogens resistant to not just the drug that targets them but to other drugs as well.
It’s a global problem that’s complicated by the diversity in use of antibiotics. In some places they can be bought over the counter; some places they are not used at all. Even in the latter locations, Manning said, studies have found traces of drug-resistant pathogens in human feces.
Farmers feed animals antibiotics. They had been used to promote growth in animals. However, as the problems emerged a ban was imposed on using any human relevant antibiotic for growth promotion.
Still they are used to treat sick animals and the antibiotics find their way into food animals, food products and in farm soil.
The soil is teeming with microbes fostering high rates of gene exchange, she said. That makes soil a fertile environment for drug resistant genes to transfer and develop more drug resistant pathogens.
One strain of deadly drug-resistant e coli emerged in Germany in 2011. It killed 54 people and caused 845 cases of kidney failure. It was eventually traced to sprouts.
Waste from pharmaceutical plants also finds its way into the environment.
At the same time, these new deadly strains are emerging “the rate of drug discovery to target bacterial infections has decreased,” Manning said.
The medical community needs to find new therapies for treating infections.
The use of probiotics to many a microbial balance in the gut is promising, she said.
Manning said people need to be “personally diligent and careful about your use of antibiotics” that means “being educated about when an antibiotic is really needed.”
As a mother of four, she’s on the frontlines. She admitted to using antibacterial soap. “But I think soap and warm water is better.”
Sometimes, even in an epic battle, the simpler weapon is the best.